X rays: From physics to clinical medicine
X rays: From physics to clinical medicine
Ibtissem Salima KAFI
We will be talking in this article about a lifesaving historical discovery that made medical miracles a routine allowing doctors to see deep inside the human body, and nearly eliminating the need of exploratory surgery. Not only this, it also revolutionized the field of physics, paving the way to many researches that contributed to our current understanding of electromagnetic radiations, radioactivity, and atomic structure.
The first Nobel Prize ever in physics was awarded in recognition of this remarkable discovery! You got it, it is about X-rays!
It all began in 1895
Wilhelm Röntgen, a German physicist, was studying cathode rays in a dark room using a Crookes tube. He noticed that a distant barium platocyanide screen was fluorescing as the cathode rays were generated! However, the screen was too far from the tube that it was impossible for cathode rays (as he understood them) to cause this fluorescence. He knew that another kind of rays might be behind this phenomenon.
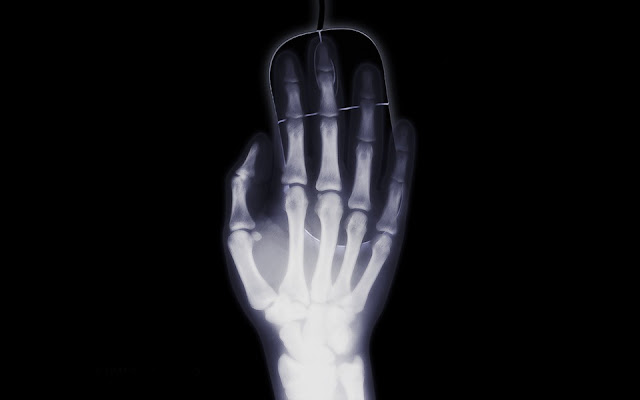
 |
Roentgen wife’s hand: first
medical x ray radiograph in history.
|
Röntgen, when later has been asked: «What have you thought at this moment? », answered: «I haven’t thought, I investigated! ». Indeed, he stood about 6 weeks in his laboratory experimenting on the new kind of rays. He found that they could expose photographic plates, like visible light. But unlike it, they could penetrate papers and salt rocks. They could not penetrate metals. He supposed they were generated as the cathode rays hit the anode. Finally, he brought his wife to his laboratory and took a photographic image of her hand using the new rays, as she saw her own bones on the film, she was shocked, and screamed: «I have seen my death! ».
Röentgen saw the great clinical applications of his discovery. He soon wrote to the Wurzburg physical medical society, announcing his findings in the famous paper «On a New kind of rays», and has named them X, for «unknown».
The world received the news with great excitement, amazed at the easiness of their production and at how these invisible rays could reveal hidden structures to our eyes. Physicians soon made diagnosis imaging using X rays an established practice in their profession. Physicists sat noticing and observing.
Much of the enthusiasm regarding the use of the yet unknown rays will be tempered as some devastating consequences started to show; severe sores, burns and cancers appeared in doctors and physicists experimenting with them. Those X-ray martyrs eventually earned international recognition, a monument at Saint Georg Hospital in Hamburg, Germany, was dedicated to them.
This caused the use of X-rays to decline, waiting for a better understanding of their nature, and a safer method to put them into practice, to be figured out.
In the meantime…
Some breakthroughs were happening in the world of physics that will soon provide the needed answers.
Henri Becquerel was a French Physicist interested in Uranium minerals. Their phosphorescent property was well known. These minerals, after being exposed to sunlight, continue to emit visible light for a period of time.
Becquerel interposed a metal between uranium salts and a photographic plate covered with dark paper (to ensure that visible light emitted from the salts won’t interfere), and put his preparation in a drawer.
The day after, he was surprised to find the image of the metal recorded on the plate!
He postulated that uranium emitted a kind of invisible rays that exposed photographic plates in a fashion similar to that seen with X rays. The difference being that Uranium rays were naturally emitted.
The new ray physics became quickly appealing to the scientific community. Marie Curie, a 28-year-old graduate physics student, chose them as a topic of research as she was preparing her doctoral thesis.
In one of her experiments, she noticed that air, which is basically a weak conductor of electric current, can conduct better if uranium salts were interposed between its particles, like if uranium rays were remotely transmitting current. She found this phenomenon of remotely transmitting energy -without a direct physical interaction- similar to what was previously discovered Radio waves capable of doing, so she gave it the name «Radioactivity».
Marie and her husband carried out more experimentations and managed to isolate two other radioactive elements; Radium and Polonium, and for that, they were awarded the Nobel Prize of physics along with Becquerel in 1903.
The new ray physics became quickly appealing to the scientific community. Marie Curie, a 28-year-old graduate physics student, chose them as a topic of research as she was preparing her doctoral thesis.
In one of her experiments, she noticed that air, which is basically a weak conductor of electric current, can conduct better if uranium salts were interposed between its particles, like if uranium rays were remotely transmitting current. She found this phenomenon of remotely transmitting energy -without a direct physical interaction- similar to what was previously discovered Radio waves capable of doing, so she gave it the name «Radioactivity».
Marie and her husband carried out more experimentations and managed to isolate two other radioactive elements; Radium and Polonium, and for that, they were awarded the Nobel Prize of physics along with Becquerel in 1903.
Rutherford in 1899 demonstrated that uranium rays were composed of three different rays: alpha, beta, and gamma, with variable penetrating capacities. He later identified beta rays to be high-speed electrons.
Marie curie then compared x rays to Gamma rays, and beta rays to cathode rays.
Doctors began noticing the ability of those rays to treat cancers, and this only motivated chemists and physicist to study them more, and helped raising the necessary funds for that.
Rutherford investigations on radiations and matter lead him to develop his famous atomic model and discover the proton and the neutron. He correctly suggested that radioactive phenomena were the result of spontaneous atomic disintegration, transforming unstable elements to more stable ones.
As for X rays, their nature remained a mystery for long. Exhibiting the properties of both waves and particles, they have always puzzled physicist who, after almost 30 years of tenacious collaborative work, settled on taking Einstein’s notion of the dual nature of electromagnetic radiations seriously. Finally, X-rays were found to be a form of electromagnetic waves, «propagated distortions of electric and magnetic fields», thus, sharing the same nature of visible light. Einstein suggested that these waves, are formed of the same elementary particles « photons » and that they only differ by the energy they carry, which is function of their wavelength/frequency.
Thus, the Radio waves we now use for communication, the microwaves we use to cook food, visible light, X-rays, and Gamma rays… although apparently very different, are in fact, of similar nature.
We will now focus on the part of these waves used in the medical field; basically X and Gamma rays, discussing how they can be produced both naturally and artificially, the way they interact with matter, and the useful and harmful consequences of these interactions.
Some Basic physics
Nuclear decay is a process whereby unstable nuclei turn to more stable ones, after emission of one of several types of radiation: Alpha radiations, Beta – “electrons”, Beta + “positrons”, Gamma rays, or X rays.
These radiations can be emitted from naturally radioactive isotopes, but in medicine, we mainly use an artificial form, either by generating synthetic radioactive isotopes, or by using X-ray tubes.
X-ray tubes are similar to Crookes tubes in that they apply a high voltage between the anode and the cathode of a vacuum tube. This accelerates electrons released from the cathode «cathode rays», and as they collide with the anode, X rays are generated.
What happens once radiations reach the body?
Electromagnetic radiations interaction with atoms varies with the energy of the photon, the atomic number, and many other characteristics. Two types of interactions between X or Gamma rays and body tissues occur in the range of energies used in clinical practice and are interesting for our discussion:
What about the image acquisition?
The sum of the above interactions is what makes possible the formation of an image when X rays photons pass through a tissue. Some of the photons are transmitted without interaction, others are absorbed, and the remaining part is scattered. As we receive the photons resulting from this «differential absorption» through a detector, we can spot pathological changes that manifest by a change in the ordinary contrast.
Types of detectors
We will now take a glimpse at how photons emitted by tissues are detected in order to form visible images.
Computed tomography, the groundbreaking innovation!
Recognized as the most significant single event in medical imaging since the discovery of X-rays, their inventors were awarded the Nobel Prize for Physiology or Medicine in 1979.
Tomography is derived from the Greek word « tomos » which means « slices », it provides cross sectional images of anatomy and allows a high degree of distinction between tissues.
With traditional radiography methods, it was impossible to visualize some organ features « for instance distinguishing brain tissue from cerebrospinal fluid », that’s because the superposition of tissues of slightly different attenuation capacities makes it hard to differentiate them on a 2D image obtained from a 3D object.
The ingenious idea of computed tomography was based on the finding of the mathematician Johann Radon who introduced the Radon transform that allows the reconstruction of the density of an unknown function, based on the data provided by its tomographic scan.
Marie curie then compared x rays to Gamma rays, and beta rays to cathode rays.
Doctors began noticing the ability of those rays to treat cancers, and this only motivated chemists and physicist to study them more, and helped raising the necessary funds for that.
Rutherford investigations on radiations and matter lead him to develop his famous atomic model and discover the proton and the neutron. He correctly suggested that radioactive phenomena were the result of spontaneous atomic disintegration, transforming unstable elements to more stable ones.
As for X rays, their nature remained a mystery for long. Exhibiting the properties of both waves and particles, they have always puzzled physicist who, after almost 30 years of tenacious collaborative work, settled on taking Einstein’s notion of the dual nature of electromagnetic radiations seriously. Finally, X-rays were found to be a form of electromagnetic waves, «propagated distortions of electric and magnetic fields», thus, sharing the same nature of visible light. Einstein suggested that these waves, are formed of the same elementary particles « photons » and that they only differ by the energy they carry, which is function of their wavelength/frequency.
Thus, the Radio waves we now use for communication, the microwaves we use to cook food, visible light, X-rays, and Gamma rays… although apparently very different, are in fact, of similar nature.
We will now focus on the part of these waves used in the medical field; basically X and Gamma rays, discussing how they can be produced both naturally and artificially, the way they interact with matter, and the useful and harmful consequences of these interactions.
Some Basic physics
Nuclear decay is a process whereby unstable nuclei turn to more stable ones, after emission of one of several types of radiation: Alpha radiations, Beta – “electrons”, Beta + “positrons”, Gamma rays, or X rays.
These radiations can be emitted from naturally radioactive isotopes, but in medicine, we mainly use an artificial form, either by generating synthetic radioactive isotopes, or by using X-ray tubes.
X-ray tubes are similar to Crookes tubes in that they apply a high voltage between the anode and the cathode of a vacuum tube. This accelerates electrons released from the cathode «cathode rays», and as they collide with the anode, X rays are generated.
What happens once radiations reach the body?
Electromagnetic radiations interaction with atoms varies with the energy of the photon, the atomic number, and many other characteristics. Two types of interactions between X or Gamma rays and body tissues occur in the range of energies used in clinical practice and are interesting for our discussion:
- The Compton scattering: It happens when a high-energy photon causes an atom to lose one of its orbital electrons, thus becoming ionized, and the remaining energy is emitted in form of a lesser energy Photon. Note that the resulting electron and photon are themselves capable of ionizing other atoms, hence the harmful effect of radiations.
- The photoelectric effect: it happens when a low energy photon causes an atom to become ionized by losing one of its orbital electrons, this time, without emitting another photon. Note that the higher the atomic number, the greater is the probability of photoelectric effect to occur. That is why bones absorb x-ray photons much greater than soft tissues do. That is also the rationale behind using contrast agents, which are products having high atomic numbers, which will artificially increase differential absorption between tissues.
What about the image acquisition?
The sum of the above interactions is what makes possible the formation of an image when X rays photons pass through a tissue. Some of the photons are transmitted without interaction, others are absorbed, and the remaining part is scattered. As we receive the photons resulting from this «differential absorption» through a detector, we can spot pathological changes that manifest by a change in the ordinary contrast.
Types of detectors
We will now take a glimpse at how photons emitted by tissues are detected in order to form visible images.
- Films: Although now becoming outdated, as widely replaced by digital detectors, the photographic films were the first photon detectors to be used in history, as we have seen.
- Digital Detectors: Simply speaking, these are devices that use the photoelectric effect «discussed earlier», to transform the signal they receive «in form of photons» into electric current. Once done, an analogic to digital converter is used to transform this voltage into a digital signal. Received by a computer, the signal serves to finally display the visible image. This principle is used in X-ray imaging, as well as in Gamma cameras (cf. applications in nuclear medicine).
Computed tomography, the groundbreaking innovation!
Recognized as the most significant single event in medical imaging since the discovery of X-rays, their inventors were awarded the Nobel Prize for Physiology or Medicine in 1979.
Tomography is derived from the Greek word « tomos » which means « slices », it provides cross sectional images of anatomy and allows a high degree of distinction between tissues.
With traditional radiography methods, it was impossible to visualize some organ features « for instance distinguishing brain tissue from cerebrospinal fluid », that’s because the superposition of tissues of slightly different attenuation capacities makes it hard to differentiate them on a 2D image obtained from a 3D object.
The ingenious idea of computed tomography was based on the finding of the mathematician Johann Radon who introduced the Radon transform that allows the reconstruction of the density of an unknown function, based on the data provided by its tomographic scan.
Therefore, the technique is about letting the X-ray beam traverse the object at multiple angles (The X-ray source rotates around the object, while the detector measuring the transmitted photons is positioned on the opposite side). A computer by the mean of reconstruction algorithms will use these measurements to assign different shades of gray to each part of the tissue, according to their degree of attenuation of the X-ray beam.
The unprecedented accuracy of the images obtained by computed tomography comes at the cost of a high radiation exposure level, but this technique led to new methods of investigation, without which it would be difficult to imagine contemporary medicine.
Applications in radiotherapy
As we have seen, radiations cause ionization of atoms. Either in form of charged particles «Alpha and B-» or photons «X and Gamma rays», the oxidative stress resulting from this type of exposure causes serious lesions in living cells, especially to the DNA molecule. Cancerous cells are the most vulnerable ones, since they are less differentiated and thus, lack the protective mechanisms necessary to repair such lesions. That’s why we can use radiotherapy to kill cancer cells while limiting damage to healthy cells. Nevertheless, a risk of developing mutations always exists.
Applications in nuclear medicine
The unprecedented accuracy of the images obtained by computed tomography comes at the cost of a high radiation exposure level, but this technique led to new methods of investigation, without which it would be difficult to imagine contemporary medicine.
Applications in radiotherapy
As we have seen, radiations cause ionization of atoms. Either in form of charged particles «Alpha and B-» or photons «X and Gamma rays», the oxidative stress resulting from this type of exposure causes serious lesions in living cells, especially to the DNA molecule. Cancerous cells are the most vulnerable ones, since they are less differentiated and thus, lack the protective mechanisms necessary to repair such lesions. That’s why we can use radiotherapy to kill cancer cells while limiting damage to healthy cells. Nevertheless, a risk of developing mutations always exists.
Applications in nuclear medicine
In contrast with conventional radiology methods where we expose the body to an external source of
radiations and receive the emitted signal, nuclear medicine is about introducing a source of radiation inside the body, in form of radiopharmaceuticals. These are synthetic radioactive elements we bind to molecules that will be used in a metabolic process. By receiving the radiation emitted by the elements through a Gamma Camera, we can localize where these metabolic processes are happening. This gives us functional, rather than anatomical clues. This is for instance used in scintigraphy, and Positron Emission Tomography (PET). While the former technique allows a two-dimensional reconstitution of the image, the last one provides a three dimensional reconstitution.
An example of radiopharmaceuticals is Technitium99 used in Scintigraphy , this is the most used radioactive element in medical imaging.
The unstable form of it «called metastable» emits a Gamma photon from its nucleus to become stable. Another example is Fluorine18, used in Positron Emission Tomography. This nucleus decays into an Oxygen nucleus by emitting a positron.
Other medical applications
Throughout history, X-rays have had various other uses. One of the most remarkable is their use in Crystallography to probe the structure of matter, based on to the difference in the way its components interact with the rays. This lead scientists to discover the structure of the DNA molecule. Besides X-rays, the low frequency Radio waves are used in Magnetic Resonance Imaging to generate anatomical images that can yield the same information as computed tomography and even better, avoiding the risk of ionizing radiations.
A Final word
Our discussion has come to an end. Through it, we tried to convey some very special events in the history of science. Beginning with an accidental discovery that was pursued by efforts of gifted scientists, eager to unravel the mysteries of nature. The cumulative interdisciplinary work of biologists, physicists, and engineers, made possible what was once unimaginable.
References
· Introduction to medical imaging: physics, engineering and clinical applications
Nadine Smith - Andrew Webb - Cambridge University Press – 2011
· An introduction to the principles of medical imaging
Chris Guy - Dominic Ffytche - Imperial College Press – 2005
. Introduction to the science of medical imaging
R. NickBryan - Cambridge University Press – 2010
. Medical imaging physics
William R.Hendee - E. RussellRitenour - 2002
. The physics of radiation therapy
Faiz M.Khan - Lippincott Williams & Wilkins - 2003
. Biological Consequences of Radiation-induced DNA Damage: Relevance to Radiotherapy
M.e. Lomax - L.k. Folkes - P. O'neill - Clinical Oncology - 2013
· Early History of X Rays: ALEXI ASSMUS
· Medical Applications of X Rays: OTHA W. LINTON
· Medscape.com: General Principles of Radiation Therapy. Author: Gary J Schreiber
An example of radiopharmaceuticals is Technitium99 used in Scintigraphy , this is the most used radioactive element in medical imaging.
The unstable form of it «called metastable» emits a Gamma photon from its nucleus to become stable. Another example is Fluorine18, used in Positron Emission Tomography. This nucleus decays into an Oxygen nucleus by emitting a positron.
Other medical applications
Throughout history, X-rays have had various other uses. One of the most remarkable is their use in Crystallography to probe the structure of matter, based on to the difference in the way its components interact with the rays. This lead scientists to discover the structure of the DNA molecule. Besides X-rays, the low frequency Radio waves are used in Magnetic Resonance Imaging to generate anatomical images that can yield the same information as computed tomography and even better, avoiding the risk of ionizing radiations.
A Final word
Our discussion has come to an end. Through it, we tried to convey some very special events in the history of science. Beginning with an accidental discovery that was pursued by efforts of gifted scientists, eager to unravel the mysteries of nature. The cumulative interdisciplinary work of biologists, physicists, and engineers, made possible what was once unimaginable.
References
· Introduction to medical imaging: physics, engineering and clinical applications
Nadine Smith - Andrew Webb - Cambridge University Press – 2011
· An introduction to the principles of medical imaging
Chris Guy - Dominic Ffytche - Imperial College Press – 2005
. Introduction to the science of medical imaging
R. NickBryan - Cambridge University Press – 2010
. Medical imaging physics
William R.Hendee - E. RussellRitenour - 2002
. The physics of radiation therapy
Faiz M.Khan - Lippincott Williams & Wilkins - 2003
. Biological Consequences of Radiation-induced DNA Damage: Relevance to Radiotherapy
M.e. Lomax - L.k. Folkes - P. O'neill - Clinical Oncology - 2013
· Early History of X Rays: ALEXI ASSMUS
· Medical Applications of X Rays: OTHA W. LINTON
· Medscape.com: General Principles of Radiation Therapy. Author: Gary J Schreiber










Commentaires
Enregistrer un commentaire